
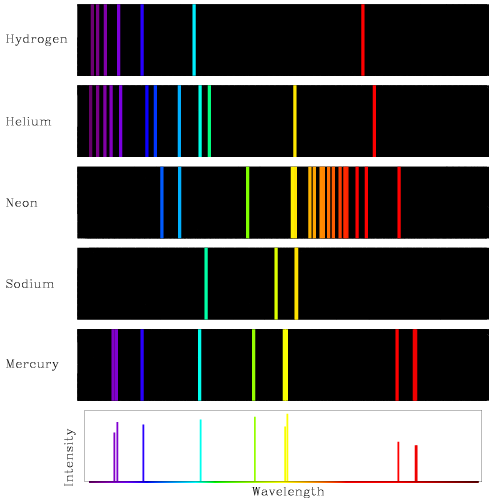
Earth's crust abundance (ppm): The concentration of an element in the Earth's crust, typically given in parts per million (ppm).Boiling point: The temperature at which a liquid substance turns into a gas.Melting point: The temperature at which a solid substance turns into a liquid.Electron configuration: The arrangement of electrons in an atom or molecule.Natural form: The most stable and abundant form of an element that occurs naturally in the environment.Atomic weight: The average mass of an element's atoms, typically given in atomic mass units (amu).K) at 25 ✬ and 1 at K) at 25✬ and 1 at 0.16 Thermal conductivity (W/cm.Symmary of properties (Xe) Atomic weight 131.293(6) Discoverer (year) Ramsay, William & Travers, Morris William (1898) Natural form gas Electron configuration 4 d 10 5 s 2 5 p 6 M.p. These spectra are usefull to identify the elements present in a sample. This results in a characteristic emission line in the spectra (which corresponds to specific wavelengths of light). When an electron in an atom is excited to a higher energy level, it can de-excite by emitting a photon of light with an energy equal to the difference between the two levels.

The orbitals are filled in a specific order, starting with the lowest energy orbital and working up.Įach element in the periodic table presents its own unique emission spectra, which is determined by the energy levels of its electrons. In the electron configuration notation, the letters "s", "p", "d", and "f" represent the different types of atomic orbitals, and the superscripts indicate the number of electrons in each orbital. The electron configuration of an element describes the arrangement of electrons in the atoms of that element, and be used to predict its chemical properties and reactivity. To propel spacecraft, xenon is used in some rocket engines that produce streams of electrified atoms that move rapidly. In food processing, xenon lamps can purify the air. The gas is harmless when inhaled and can be used as an anesthetic. It glows brightly when electrified, making it useful in very powerful lamps, such as those used in film projectors and automobile headlights. Like the other noble gases, xenon is colorless and odorless. (orig.Xenon is so rare that there is only one atom of this gaseous element for every 10 million atoms in the air. A list of wavelengths for observed laser transitions showing the present classifications and a discussion of the determination of the ionization potential of Xe II concludes the paper. The latter are compared to recent experimental measurements. We present an analysis of the 5s photoelectron satellite spectrum of Xe based on our calculated eigenvector compositions and calculations of transition probabilities for ground state transitions as well as lifetimes for the 6p levels. The former is the better coupling scheme for Xe II. The levels have been named in jK and for many levels also in LS coupling. A least-squares fit to the 5p 46p configuration is also reported. We have carried out least-squares fits to the even configurations and report the resulting parameter values and eigenvector compositions. Also a number of g-factors have been determined for the first time and we give in total 75 g-factors. We report 161 energy levels which have been identified on the basis of classifications of 950 lines. The latter has kindly placed the original wavelength list covering the wavelength range 10220-390 A at our disposal. This spectrum has been reanalyzed on the basis of the wavelength material published by Drs J. We present a revised analysis of the spectrum of singly ionized xenon, Xe II.


 0 kommentar(er)
0 kommentar(er)
